What Does Purma Biologics, Llc Do?
Table of Contents10 Simple Techniques For Purma Biologics, LlcEverything about Purma Biologics, LlcPurma Biologics, Llc Can Be Fun For AnyoneGetting My Purma Biologics, Llc To Work
Also if the contamination is eliminated, there is no way of making sure that the resulting cell line will certainly have the very same attributes as the first one due to the stress and anxiety of the treatment.
Bovine-derived items also may have the agent in charge of bovine spongiform encephalopathy (BSE). Sadly, there is no test for the existence of this representative and we extremely suggest that you acquire all bovine items (consisting of sera) from nations not influenced by BSE such as the United States, Australia, and New Zealand.
Almost all lotions today are filteringed system with several 0. Further, each great deal is examined for its capability to support cell development and is the exact same sera made use of in ATCC laboratories.
Unknown Facts About Purma Biologics, Llc
Do not store sera at temperature levels over 20C for any size of time. The adhering to procedure is utilized to thaw lotion: Place icy product in a fridge at 2C to 8C over night.
Defrosting lotion in a bath above 40C without mixing may cause the formation of a precipitate inside the container. All products might keep some fibrinogen. Because external factors might launch the conversion of fibrinogen to fibrin, flocculent material or turbidity might be observed after serum is defrosted. The visibility of this material does not alter the product's efficiency.

A precipitate can form in product when incubated at 37C or greater for extended durations of time which might be misinterpreted for microbial contamination. Heat inactivation of products can also create the formation of precipitates.
Warm inactivation is normally unnecessary and can be damaging to the development of some cells - https://lwccareers.lindsey.edu/profiles/3830110-stephen-flack. It will certainly reduce or destroy development elements existing in the serum. Warm inactivation was initially carried out to suspend complement (a team of proteins present in products that become part of the immune feedback) as well as to ruin mycoplasma contaminants
The Best Strategy To Use For Purma Biologics, Llc
Use sufficient water to immerse the bottle above the level of serum. Mix thawed lotion by mild inversion and area in the 56C bathroom.
Remove serum from water bathroom, awesome rapidly (sluggish cooling can often turn around the inactivation of enhance activity), and store at 20C or chillier. Acid base indicator.
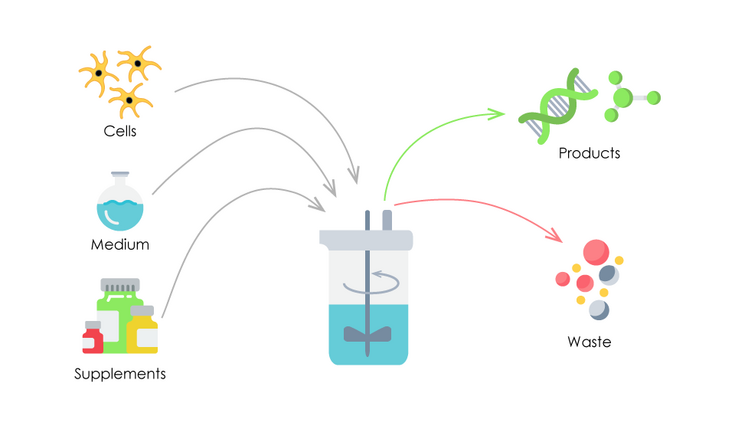
It can also be developed to aid control ecological conditions, such as osmotic pressure and p, H. Unlike microbiological culture media, cell society media are usually a lot more intricate because of the reality that cells originated from entire microorganisms typically can not expand without the addition of, as an example, hormonal agents or development elements which normally take place in vivo.
Things about Purma Biologics, Llc
The p, H degree is identified by matching the shade of the solution to a shade graph (Cell culture media). Antibiotics: Prescription antibiotics are often made use of in cell click society as a means to protect against contamination, preserve aseptic conditions, or choose for cells including hereditary adjustments.
According to the Method of Prep work Ready-to-use medium is a strong or liquid tool supplied in ready-to-use kind or ready-to-use after reflow and supplementation. Tool prepared from dehydrated solutions is purchased in completely dry form and needs rehydration and handling before usage, resulting in either a total tool or an incomplete medium to which supplements are included before usage.
Recognition of critical parts: As soon as an ideal base media has been identified, it is crucial to figure out which parts are critical for growth and productivity. This can be achieved by systematically varying the concentration of individual components and observing the effect on cell growth and productivity. Optimization of vital elements: Based upon the outcomes gotten in step 2, the concentrations of critical components can be optimized to achieve maximum growth and productivity.
Validation of optimal conditions: Once the ideal conditions have been determined, it is very important to verify them by repeating the experiments and verifying that the very same results are acquired. Refine scale-up: After the bioprocess media has been enhanced, the process can be scaled up from laboratory to industrial range, guaranteeing that the optimal problems for development and performance are preserved throughout.